Abstract
Rationale: Donor cell leukemia (DCL) is formally defined as the new onset of hematological malignancy following an allogeneic hematopoietic stem cell transplantation (alloHSCT) that is derived from the donor. DCL is a rare but increasingly recognized complication with more than 100 cases being reported in the literature and an incidence between 0.12 to 5% (1-6). The identification of DCL is crucial in order to determine the most appropriate therapy for the patient. The underlying mechanisms that contribute to DCL remain largely unknown with no formal guidelines pertaining to prevention.
Objective: To determine the incidence of DCL in a patient cohort and better characterize this patient population in terms of natural history, response to therapy, clinical course, and outcomes.
Methods: A retrospective chart review was conducted to identify DCL cases that were diagnosed between 2012 to 2022 at the Juravinski Cancer Center (JCC) in Hamilton, Ontario. All patients were 18 years of age or older and diagnosed with a hematological malignancy that was treated with a sex mismatched alloHSCT. DCL was defined as a new presentation of hematological malignancy as per the 2022 WHO classification (7), with the source being from the donor, as confirmed by chimerism status testing with short tandem repeat polymerase chain reaction (STR-PCR) and karyotyping.
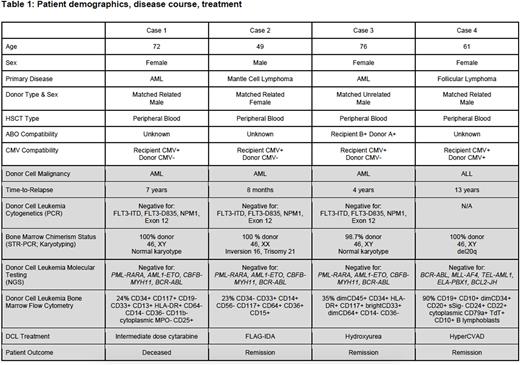
Results: We identified four patients with confirmed DCL (Table 1). These patients previously received a sex mismatched alloHSCT between 2005 to 2017. A total of 205 patients at JCC received a sex mismatched alloHSCT during the same time span of 2005 to 2017. The incidence of DCL in this cohort was 1.95%. Three patients developed AML and one patient had B-ALL. Three patients were female, and one was male, with a median age of 64.5 years. Three cases were from sibling-matched donors. All patients had normal clonal cell next generation sequencing using a NN-gene myeloid panel (OGT), whereas two patients had an abnormal donor clonal karyotype (inversion 16, deletion 20q, trisomy 21). The latency period between initial malignancy and DCL was between 8 months to 13 years post-transplant. Three patients were alive at time of chart review, with a 25% mortality rate.
Conclusions: The time span of the DCL latency period is broad, ranging from months to upwards of a decade. In patients with leukemia diagnosed following sex mismatched alloHSCT, regardless of when it occurs following the initial malignancy, both relapse of leukemia and DCL should be considered as potential differentials. DCL may be the product of somatic and/or germline mutations that warrants additional genetic testing and counselling prior to transplantation. The concept of more comprehensive molecular and cytogenetic testing to identify somatic and germline mutations in related donors prior to alloHSCT to assist with prevention of DCL needs to be explored further.
References:
1. Deshmukh KG, Kelemen K. Lessons Learned from Donor Cell-Derived Myeloid Neoplasms: Report of Three Cases and Review of the Literature. Life (Basel). 2022;12(4):559.
2. Suping Zhang, Li Li, Weijie Cao, et al. Donor cell leukemia/myelodysplastic syndrome after allogeneic stem cell transplantation: a rare phenomenon with more challenges for hematologists. Hematology. 2021;26:1:648-651.
3. Hertenstein B, Hambach L, Bacigalupo A, et al. Development of leukemia in donor cells after allogeneic stem cell transplantation - a survey of the European Group for Blood and Marrow Transplantation (EBMT). Haematologica. 2005;90:969-975.
4. Hayes C, Petersen B, Malone A. Donor cell myeloid sarcoma in an umbilical cord transplant patient: A case report and a review of the literature. Case Rep Hematol. 2015:186869.
5. Wiseman DH. Donor cell leukemia: a review. Biol Blood Marrow Transplant. 2011;17(6):771-789.
6. Gondek LP, Zheng G, Ghiaur G, et al. Donor cell leukemia arising from clonal hematopoiesis after bone marrow transplantation. Leukemia. 2016;30(9):1916-1920.
7. Khoury JD, Solary E, Abla O. et al. The 5th edition of the World Health Organization Classification of Haematolymphoid Tumours: Myeloid and Histiocytic/Dendritic Neoplasms. Leukemia. 2022;36:1703-1719.
Disclosures
Raslan:Pfizer: Honoraria. Leber:Pfizer: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; AMGEN: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; BMS: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Abbvie: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Berg:Imago Biosciences: Research Funding; Celgene: Other: Travel Support; Astellas: Other: Travel Support; Alexion: Other: Travel Support; Abbvie: Other: Travel Support; Incyte: Honoraria; Riemser Pharma GmbH: Consultancy; Jazz Pharmaceuticals: Consultancy; AVIR Pharma: Consultancy; Roche: Honoraria; Takeda: Honoraria. Khalaf:Novartis: Honoraria; Pfizer: Honoraria; Paladin: Honoraria; Astellas: Honoraria; Jazz: Honoraria; Taiho: Consultancy, Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal